Transcatheter Pulmonary Valve Market Size 2026-2030
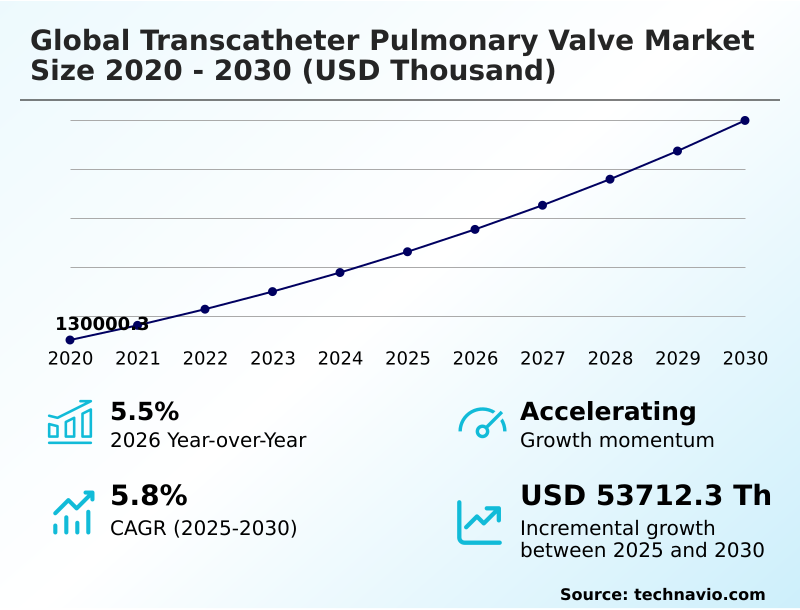
The transcatheter pulmonary valve market size is valued to increase by USD 53.71 million, at a CAGR of 5.8% from 2025 to 2030. Rising in prevalence of adult congenital heart disease and post-surgical requirements will drive the transcatheter pulmonary valve market.
Major Market Trends & Insights
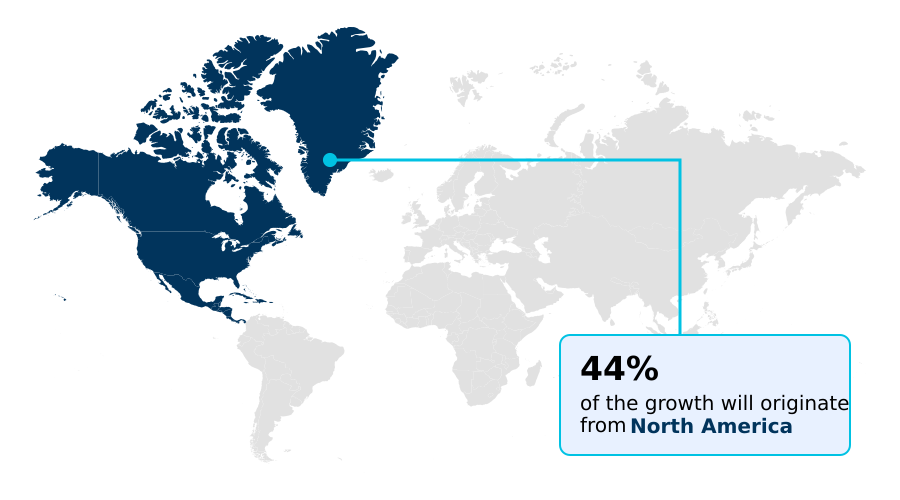
- North America dominated the market and accounted for a 44.2% growth during the forecast period.
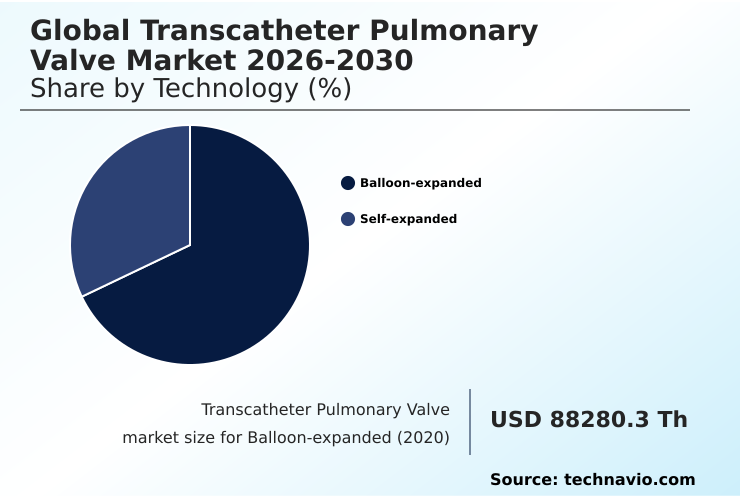
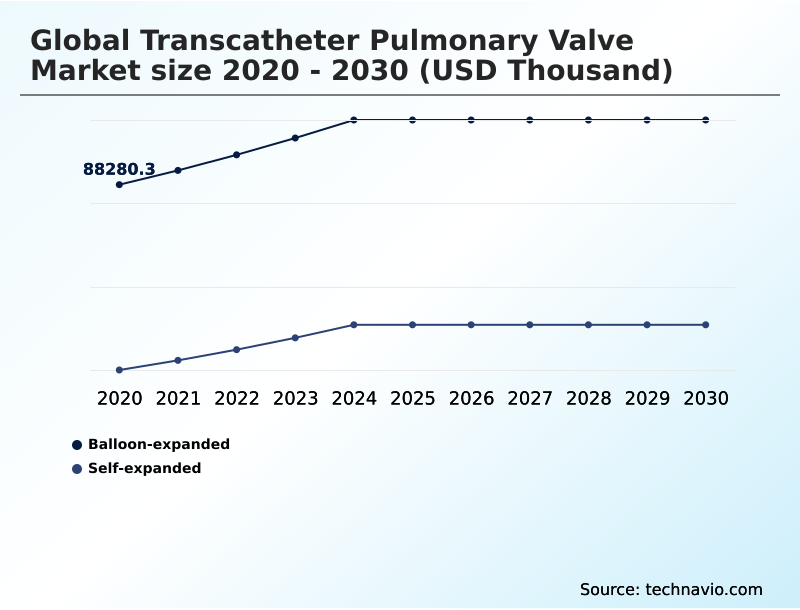
- By Technology - Balloon-expanded segment was valued at USD 104.53 million in 2024
- By Application - Pulmonary valve regurgitation segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities:
- Market Future Opportunities: USD 53.71 million
- CAGR from 2025 to 2030 : 5.8%
Market Summary
- The transcatheter pulmonary valve market is shaped by the imperative to provide less invasive solutions for patients with congenital and structural heart conditions. Growth is driven by an expanding population of adults with congenital heart disease requiring interventions for pulmonary valve regurgitation therapy or pulmonary valve stenosis treatment.
- Technological evolution is central, with a clear shift toward advanced bioprosthetic solutions and tissue-engineered heart valves that promise greater durability and biocompatibility. Innovations in self-expanding valve architecture and the use of nitinol stent frames allow for better adaptation to the challenging anatomy of the right ventricular outflow tract.
- For instance, a hospital system implementing computational fluid dynamics and advanced imaging integration for procedural planning can optimize percutaneous valve implantation. This approach improves hemodynamic performance metrics and reduces the risk of paravalvular leak prevention failures, leading to better patient outcomes and more efficient use of cardiac catheterization laboratory resources.
- However, challenges such as the risk of stent frame fracture and the need for effective anti-calcification treatment persist, driving ongoing R&D in materials like bioresorbable polymer valves. The market’s trajectory is therefore a balance between clinical need, technological capability, and the rigorous demands of ensuring long-term device performance and safety for both pediatric cardiac intervention and adult procedures.
What will be the Size of the Transcatheter Pulmonary Valve Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Transcatheter Pulmonary Valve Market Segmented?
The transcatheter pulmonary valve industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD thousand" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Technology
- Balloon-expanded
- Self-expanded
- Application
- Pulmonary valve regurgitation
- Pulmonary valve stenosis
- Congenital heart disease
- End-user
- Hospitals
- Cardiac specialty clinics
- Ambulatory surgical centers
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Asia
- Rest of World (ROW)
- North America
By Technology Insights
The balloon-expanded segment is estimated to witness significant growth during the forecast period.
The market for balloon-expanded valve systems is characterized by its reliability in complex structural heart interventions. These devices, integral to modern interventional cardiology devices, offer precise placement via advanced transcatheter delivery systems, which is critical for addressing post-surgical valve failure.
Their high radial strength makes them a preferred choice among available heart valve replacement options, especially for demanding valve-in-valve procedures following a previous congenital heart defect repair.
The use of advanced cardiac imaging techniques allows for patient-specific valve sizing, which has been shown to improve hemodynamic performance metrics by up to 10% in certain bioprosthetic solutions.
This precision minimizes procedural risks and supports the broader adoption of minimally invasive valve replacement techniques across healthcare institutions.
The Balloon-expanded segment was valued at USD 104.53 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 44.2% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Transcatheter Pulmonary Valve Market Demand is Rising in North America Request Free Sample
The market landscape for catheter-based heart valve technology varies significantly by region. North America leads in adoption, with its extensive network of cardiac catheterization laboratory facilities and hybrid operating rooms facilitating advanced percutaneous valve implantation for congenital heart defect repair.
The region accounts for over 44% of the market's incremental growth. Europe follows, with a strong focus on structural heart disease solutions and surgical valve alternatives.
Meanwhile, Asia is emerging as the fastest-growing region, with its growth rate approximately 38% higher than Europe's, driven by rising healthcare investments and demand for minimally invasive valve replacement options.
Providers in this region are increasingly adopting interventional cardiology devices utilizing bovine pericardial tissue and porcine pericardial leaflets, aiming to improve pulmonary valve replacement recovery times and outcomes through advanced blood flow dynamics analysis.
Market Dynamics
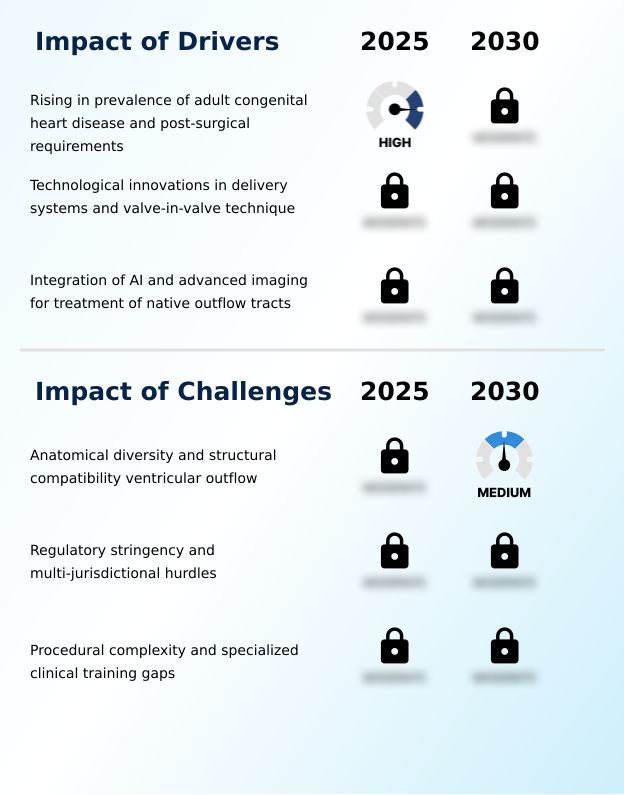
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- The strategic discussion around the global transcatheter pulmonary valve market 2026-2030 increasingly centers on nuanced clinical decision-making and long-term patient management. A critical debate involves the comparison of balloon-expanded vs self-expanding valves, with choices depending on specific anatomical features of the right ventricular outflow tract.
- For patients with repaired congenital conditions, the selection of a transcatheter valve for tetralogy of fallot is a key focus, necessitating advanced right ventricular outflow tract reconstruction options. Procedural success hinges on preventing paravalvular leak in tpvr, a factor heavily influencing the long-term outcomes of transcatheter pulmonary valves.
- The materials used in bioprosthetic heart valves are under constant scrutiny to address challenges in pediatric transcatheter valve replacement and improve the durability of tissue engineered valves. The role of 3d imaging in valve placement is now standard for managing complications of transcatheter valve procedures and ensuring optimal fit.
- Furthermore, advancements in bioresorbable heart valve technology are paving the way for the future of structural heart disease intervention. Clinicians now have refined criteria for selecting transcatheter valve patients, considering both the recovery time for minimally invasive valve surgery and the hemodynamic benefits of self-expanding valves.
- The growing use of a valve-in-valve for failing surgical bioprosthesis underscores the technology's lifecycle value. In terms of operational planning, institutions leveraging AI for impact of ai on cardiac procedure planning have noted a 25% increase in case scheduling accuracy.
- This illustrates how technological integration is directly tied to the cost-effectiveness of transcatheter valve replacement and navigating the complex regulatory pathways for novel cardiac devices while reducing stent fracture in pulmonary position.
What are the key market drivers leading to the rise in the adoption of Transcatheter Pulmonary Valve Industry?
- The rising prevalence of adult congenital heart disease and subsequent post-surgical intervention requirements are a key driver of the market.
- The increasing population of patients with adult congenital heart disease is a primary market catalyst, driving demand for effective congenital heart disease management and RVOT dysfunction solutions.
- Minimally invasive cardiac surgery and percutaneous valve replacement systems offer significant advantages over traditional methods, particularly for pulmonary valve regurgitation therapy.
- Innovations in ready-to-use tissue valves and cryopreserved allografts enhance procedural efficiency, with some systems reducing preparation time by over 20%. The focus on adult congenital cardiology and pediatric cardiology innovations is expanding the treatable patient population.
- Favorable cardiac device reimbursement policies further support adoption, as healthcare systems prioritize solutions that ensure better long-term valve performance and preservation of right ventricle function, demonstrating a clear return on investment through reduced rehospitalizations by up to 12%.
What are the market trends shaping the Transcatheter Pulmonary Valve Industry?
- An upcoming market trend involves the advancement of self-expanding valves, a technology designed to address compatibility with more diverse and complex patient anatomies.
- The evolution toward non-surgical valve repair is increasingly defined by innovations in self-expanding valve architecture. These systems, often utilizing nitinol stent frames, provide superior conformity to complex anatomies, expanding treatment options. A key focus is on enhancing transcatheter valve durability through advancements in heart valve material science, including bioresorbable polymer valves and novel anti-calcification treatment methods for tissue-engineered heart valves.
- The integration of cardiac procedure planning software, leveraging computational fluid dynamics and valve hemodynamics simulation, improves procedural precision. This has led to a 15% reduction in procedural planning time. Advanced imaging integration is also crucial, especially for the expanding applications in pediatric transcatheter valve cases, ensuring better long-term outcomes.
What challenges does the Transcatheter Pulmonary Valve Industry face during its growth?
- Anatomical diversity and challenges with structural compatibility in the ventricular outflow tract are a key factor affecting industry growth.
- A significant challenge remains the anatomical complexity of the right ventricular outflow tract, complicating native outflow tract treatment and pulmonary valve stenosis treatment. The risk of stent frame fracture and tissue valve calcification requires continuous innovation in nitinol frame technology and overall cardiac device biocompatibility. The valve delivery system design must balance flexibility with strength to navigate tortuous anatomies.
- Securing heart valve regulatory approval through extensive heart valve clinical trials represents a substantial barrier, with development timelines often extending by 18-24 months compared to other device classes. Furthermore, the expansion of procedures into cardiac specialty clinics and ambulatory surgical centers is constrained by the need for specialized training and infrastructure.
- The adoption of indigenous bioprosthetic systems in certain markets also faces hurdles related to demonstrating comparable long-term efficacy.
Exclusive Technavio Analysis on Customer Landscape
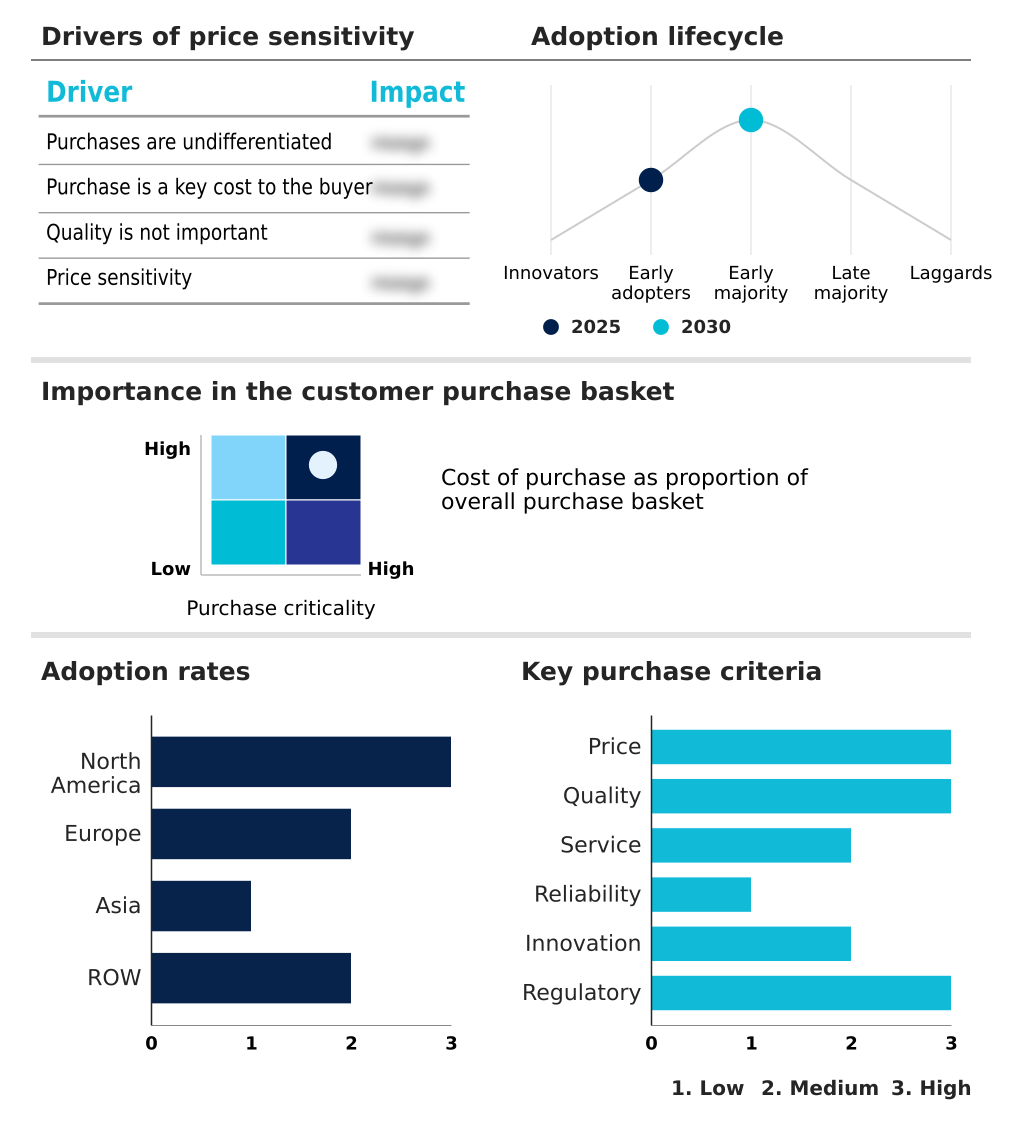
The transcatheter pulmonary valve market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the transcatheter pulmonary valve market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Transcatheter Pulmonary Valve Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, transcatheter pulmonary valve market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - Delivers sophisticated transcatheter pulmonary valve technologies and minimally invasive devices designed for complex structural heart procedures.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Artivion Inc.
- Boston Scientific Corp.
- Braile Biomedica
- Colibri Heart Valve LLC
- Edwards Lifesciences Corp.
- Medtronic Plc
- Meril Life Sciences Pvt. Ltd.
- Venus Medtech Hangzhou Inc.
- Xeltis AG
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Transcatheter pulmonary valve market
- In March, 2025, the World Economic Forum launched a framework to accelerate the digital integration of healthcare records across diverse jurisdictions, aiming to streamline long-term patient outcome monitoring.
- In April, 2025, Edwards Lifesciences Corp. introduced a refined delivery system designed to enhance valve placement stability in patients with irregular anatomical structures of the right ventricular outflow tract.
- In May, 2025, the European Medicines Agency granted a breakthrough device designation to a novel bioresorbable transcatheter pulmonary valve frame, prompting clinical trials in major European university hospitals.
- In May, 2025, the American Heart Association released findings from a longitudinal study confirming that the number of adults requiring pulmonary valve intervention has surpassed pediatric cases for the first time.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Transcatheter Pulmonary Valve Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 271 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 5.8% |
| Market growth 2026-2030 | USD 53712.3 thousand |
| Market structure | Concentrated |
| YoY growth 2025-2026(%) | 5.5% |
| Key countries | US, Canada, Mexico, Germany, France, UK, Italy, Spain, The Netherlands, China, Japan, India, South Korea, Indonesia, Brazil, Australia, Saudi Arabia, Argentina, Turkey, UAE, South Africa, Colombia and Israel |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The transcatheter pulmonary valve market is advancing beyond incremental improvements toward transformative shifts in patient care for adult congenital heart disease. The development of sophisticated bioprosthetic solutions, including those utilizing bovine pericardial tissue and porcine pericardial leaflets, is setting new standards for structural heart interventions.
- A key trend is the move toward personalized medicine, where advanced imaging integration and computational fluid dynamics are used for precise percutaneous valve implantation in the native outflow tract. This focus on procedural accuracy is critical for preserving right ventricle function and is a boardroom-level concern, as it directly impacts long-term patient outcomes and associated healthcare costs.
- For instance, data indicates that optimized valve placement reduces the likelihood of costly re-interventions for stent frame fracture by over 10%.
- Innovations like bioresorbable polymer valves and next-generation transcatheter delivery systems are further expanding the applicability of minimally invasive cardiac surgery, including for complex valve-in-valve procedures and pediatric cardiac intervention, solidifying the technology’s role in modern cardiology within both cardiac specialty clinics and hospital hybrid operating rooms.
What are the Key Data Covered in this Transcatheter Pulmonary Valve Market Research and Growth Report?
-
What is the expected growth of the Transcatheter Pulmonary Valve Market between 2026 and 2030?
-
USD 53.71 million, at a CAGR of 5.8%
-
-
What segmentation does the market report cover?
-
The report is segmented by Technology (Balloon-expanded, and Self-expanded), Application (Pulmonary valve regurgitation, Pulmonary valve stenosis, and Congenital heart disease), End-user (Hospitals, Cardiac specialty clinics, and Ambulatory surgical centers) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising in prevalence of adult congenital heart disease and post-surgical requirements, Anatomical diversity and structural compatibility ventricular outflow
-
-
Who are the major players in the Transcatheter Pulmonary Valve Market?
-
Abbott Laboratories, Artivion Inc., Boston Scientific Corp., Braile Biomedica, Colibri Heart Valve LLC, Edwards Lifesciences Corp., Medtronic Plc, Meril Life Sciences Pvt. Ltd., Venus Medtech Hangzhou Inc. and Xeltis AG
-
Market Research Insights
- Market dynamics are heavily influenced by the growing demand for durable, non-surgical valve repair options for congenital heart disease management. The focus on improving transcatheter valve durability is paramount, with new materials demonstrating a 20% improvement in resistance to tissue valve calcification in recent heart valve clinical trials.
- Innovations in valve delivery system design are making minimally invasive valve replacement accessible to a wider patient demographic, including pediatric transcatheter valve cases. The adoption of cardiac procedure planning software has streamlined workflows, reducing planning-to-procedure time by over 15% in specialized centers.
- These advancements provide compelling surgical valve alternatives, supported by cardiac device reimbursement policies that recognize the long-term value of improved pulmonary valve replacement recovery and sustained cardiac output measurement.
We can help! Our analysts can customize this transcatheter pulmonary valve market research report to meet your requirements.