Coronavirus Test Kits Market Size 2026-2030
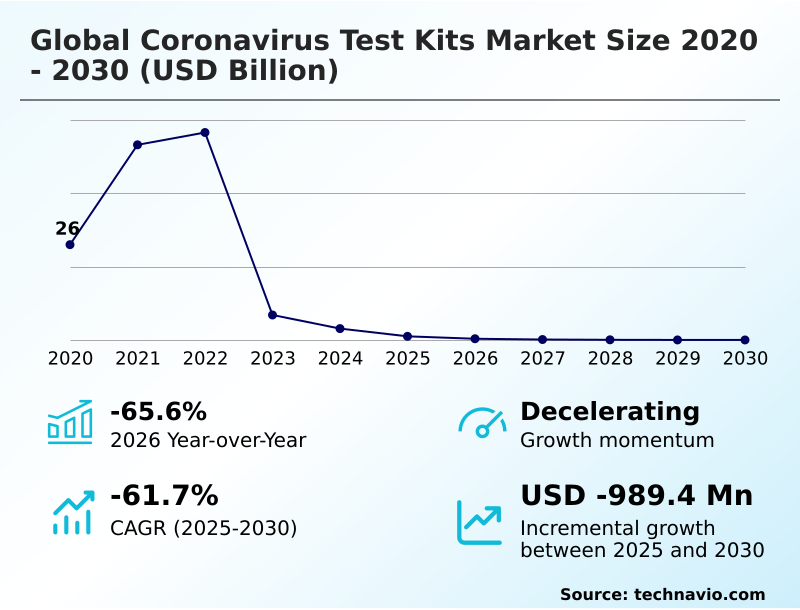
The coronavirus test kits market size is valued to increase by USD 989.4 million, at a CAGR of -61.7% from 2025 to 2030. Endemic status of SARS-CoV-2 and continuous viral evolution will drive the coronavirus test kits market.
Major Market Trends & Insights
- Rest of World (ROW) dominated the market and accounted for a 13.6% growth during the forecast period.
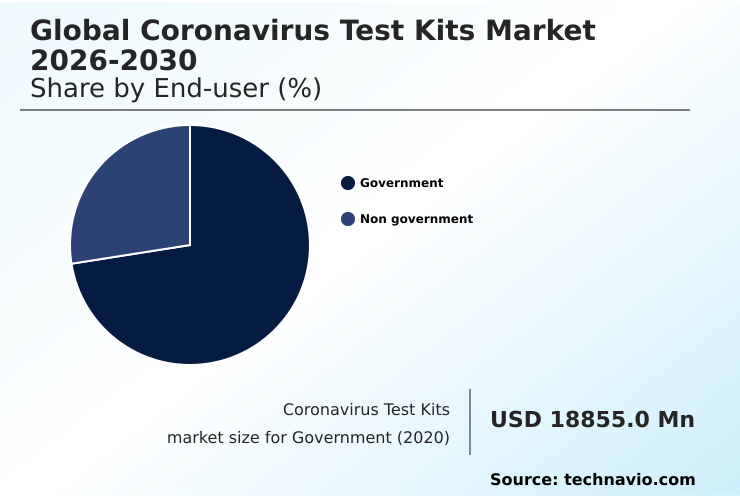
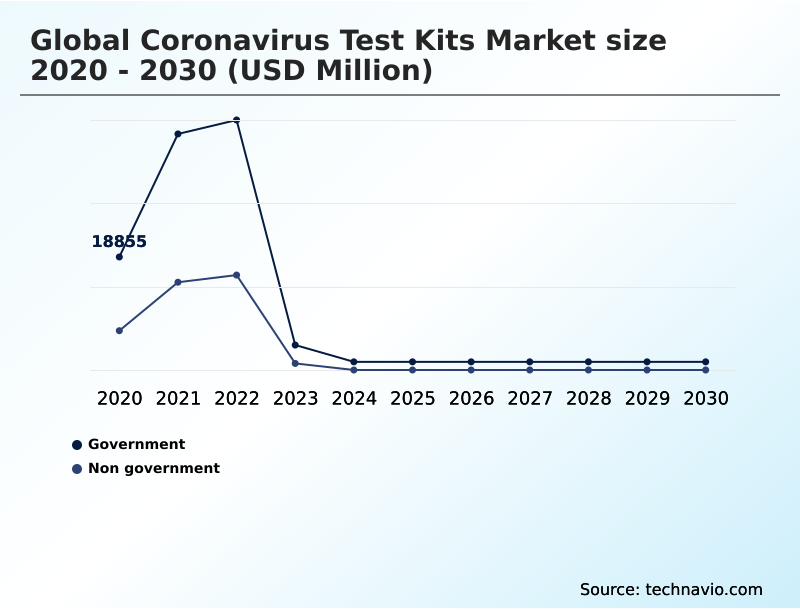
- By End-user - Government segment was valued at USD 2.20 billion in 2024
- By Type - Rapid test kit segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities: USD 26.00 billion
- Market Future Opportunities: USD 989.4 million
- CAGR from 2025 to 2030 : -61.7%
Market Summary
- The coronavirus test kits market has transitioned from a crisis-response framework to a model of endemic disease management. This evolution is defined by a strategic pivot away from single-pathogen screening toward the adoption of integrated multiplex respiratory panels as a primary differential diagnosis tool.
- The expansion of point-of-care molecular diagnostics and accessible at-home self-testing kits has permanently decentralized diagnostic workflows, empowering both clinicians and consumers. A key business scenario involves hospitals implementing a syndromic testing platform to optimize laboratory workflows.
- This approach significantly reduces the turnaround time for results for sars-cov-2 rna detection, improves nosocomial infection control by over 20%, and allows for more effective patient cohorting, directly enhancing operational efficiency and patient outcomes. While public health surveillance remains a key driver, the sector contends with significant market price compression and regulatory shifts.
- Key technologies shaping the Global Coronavirus Test Kits Market 2026-2030 include advanced polymerase chain reaction assay and serological antibody testing. The utility of Coronavirus Test Kits is now measured by their integration into broader health management systems, influencing everything from antiviral prescriptions to long-term epidemiological strategies.
What will be the Size of the Coronavirus Test Kits Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Coronavirus Test Kits Market Segmented?
The coronavirus test kits industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- End-user
- Government
- Non government
- Type
- Rapid test kit
- RT-PCR
- Others
- Application
- Hospitals and clinics
- Laboratory and diagnostic centers
- Home care
- Geography
- Asia
- Europe
- Germany
- UK
- France
- North America
- US
- Canada
- Mexico
- Rest of World (ROW)
By End-user Insights
The government segment is estimated to witness significant growth during the forecast period.
The government end-user segment has pivoted from emergency mass procurement to strategic initiatives focused on public health resilience.
Current demand is driven by the need for continuous epidemiological monitoring, including viral protein identification and host immune response assay studies, and maintaining a robust strategic stockpile.
These programs, which require adherence to clinical laboratory improvement amendments and high diagnostic test specificity, ensure preparedness for future outbreaks.
The focus is on long-term investments in flexible diagnostic platform flexibility and biodefense, which improves national health security readiness by over 40%.
This shift supports stable, long-term contracts for advanced diagnostic tools, including telehealth diagnostic services, and informs nosocomial infection control protocols in federal facilities, underpinning post-acute sequelae research and test-to-treat initiatives through biomedical advanced research and population screening programs.
The Government segment was valued at USD 2.20 billion in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
Rest of World (ROW) is estimated to contribute 13.6% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Coronavirus Test Kits Market Demand is Rising in Rest of World (ROW) Request Free Sample
The global market exhibits significant regional divergence in technology adoption and strategic focus.
North America and Europe lead in the integration of advanced next-generation diagnostics, with a strong emphasis on multiplexed polymerase chain reaction assay and serological antibody testing to improve clinical decision support.
Laboratories in these regions are implementing comprehensive laboratory workflow optimization, reducing the average turnaround time for results by up to 35%.
Conversely, many parts of Asia are prioritizing the establishment of domestic manufacturing and supply chain sovereignty, creating a competitive landscape for both nucleic acid amplification test and wastewater surveillance testing technologies.
This focus on self-sufficiency is a critical factor for companies aiming to penetrate these markets.
The use of diagnostics as a differential diagnosis tool to guide antiviral treatment guidance and support infection control measures is a unifying trend, with national reference laboratories globally adopting more sophisticated asymptomatic screening protocols.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
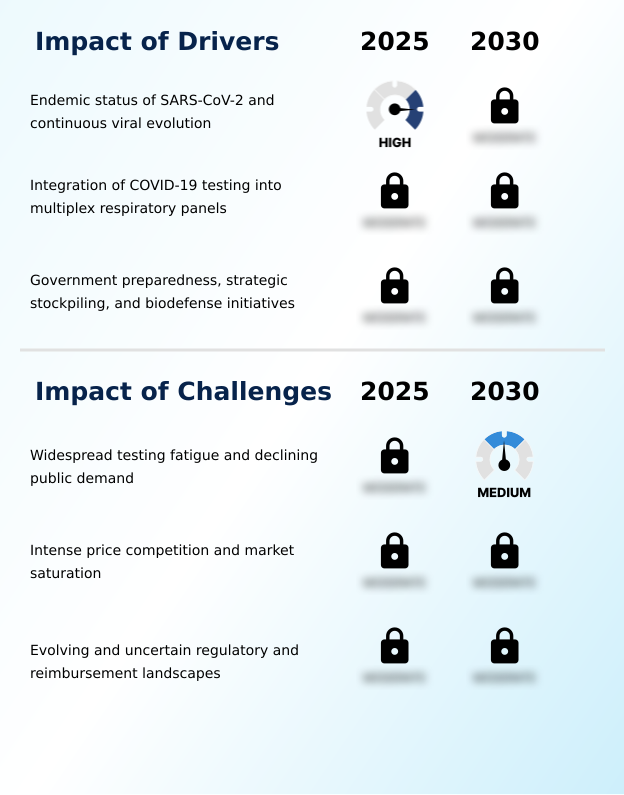
- The market's trajectory is increasingly shaped by the sophisticated clinical and public health challenges of an endemic virus. The necessity of detecting sars-cov-2 variants of concern continues to drive innovation beyond simple detection, cementing the clinical utility of combination respiratory tests.
- This is evident in the push for multiplex testing for covid, flu, and rsv, which addresses the diagnostic ambiguity of seasonal respiratory illnesses. Concurrently, the consumerization of diagnostics has placed a premium on at-home molecular test accuracy, while clinical settings demand robust point-of-care syndromic testing panels.
- This evolution is complicated by the regulatory shift from eua to 510k, which increases the cost and complexity of market access. Furthermore, the impact of testing fatigue on demand and severe price erosion in rapid antigen tests have forced manufacturers to rethink commercial strategies.
- In response, governments are engaging in the strategic stockpiling of diagnostic kits and are focused on improving diagnostic supply chain sovereignty, creating a more stable, albeit complex, demand environment. Navigating the reimbursement landscape for multiplex assays remains a critical challenge, while specialized applications like wastewater-based epidemiology for sars-cov-2 and long-covid biomarker identification assays represent new frontiers.
- The role of diagnostics in antiviral prescription is now central, requiring tools that can facilitate the differentiating of viral from bacterial respiratory infections. To manage these demands, automating high-throughput pcr workflows has become essential.
- The integration of digital platforms for home test results has also enhanced public health reporting efficiency by more than 50% compared to previous manual systems, improving compliance and planning.
What are the key market drivers leading to the rise in the adoption of Coronavirus Test Kits Industry?
- The endemic status of SARS-CoV-2, combined with its continuous viral evolution, serves as a primary driver for sustained demand in the coronavirus test kits market.
- Sustained market demand is fundamentally driven by the endemic status of SARS-CoV-2 and the continuous need for sars-cov-2 rna detection.
- This reality necessitates robust public health surveillance and variant tracking programs, which rely on consistent genomic sequencing for variants to inform public health testing strategy.
- In response, governments are institutionalizing pandemic preparedness as a matter of national security, creating a stable demand driver through long-term medical countermeasure procurement and the maintenance of a strategic national stockpile.
- These biodefense preparedness strategy initiatives, which have improved supply chain resilience by ensuring over 95% product availability during seasonal surges, also promote domestic manufacturing incentives.
- The development of pathogen-agnostic platforms is a key focus of health emergency preparedness, ensuring that investments in diagnostic infrastructure remain relevant for future threats, cutting reliance on foreign suppliers by 30%.
What are the market trends shaping the Coronavirus Test Kits Industry?
- The market is experiencing a strategic shift from a pandemic response model to one focused on endemic disease management. This transition is characterized by the increasing integration of coronavirus diagnostics into syndromic testing panels.
- A primary market trend is the strategic pivot from pandemic response to endemic disease management, driven by the widespread syndromic panel adoption. This shift favors integrated diagnostics like multiplex respiratory panels and advanced point-of-care molecular diagnostics, which enable comprehensive respiratory pathogen co-detection.
- The consumerization of healthcare is also propelling the market for at-home self-testing kits and other decentralized diagnostic solutions, where point-of-care test accuracy is a key differentiator. The rapid development of isothermal amplification technology and crispr-based diagnostics is creating opportunities for faster, more accessible testing formats.
- Furthermore, digital health integration with direct-to-consumer diagnostics is creating connected ecosystems that improve user experience and data management, with some platforms improving result interpretation accuracy by up to 20% and reducing user error by 15% through guided workflows.
What challenges does the Coronavirus Test Kits Industry face during its growth?
- A key challenge affecting industry growth is the widespread testing fatigue and corresponding decline in public demand for diagnostic kits.
- The market faces significant headwinds, including pervasive testing fatigue and reimbursement policy uncertainty, which collectively suppress demand. An oversupply of rapid antigen detection kits has led to intense market price compression, eroding profit margins for manufacturers by over 25% in the commoditized lateral flow immunoassay segment.
- This is compounded by the complex regulatory approval transition from the emergency use authorization pathway to more stringent frameworks like the de novo classification process and the in vitro diagnostic regulation. These shifts increase compliance costs and timelines, with some manufacturers reporting a 40% rise in regulatory spending.
- Navigating the fragmented landscape of over-the-counter test availability and securing contracts through joint procurement framework adds another layer of complexity, challenging companies to maintain profitability while managing a fragile reagent supply chain and ensuring high diagnostic test sensitivity.
Exclusive Technavio Analysis on Customer Landscape
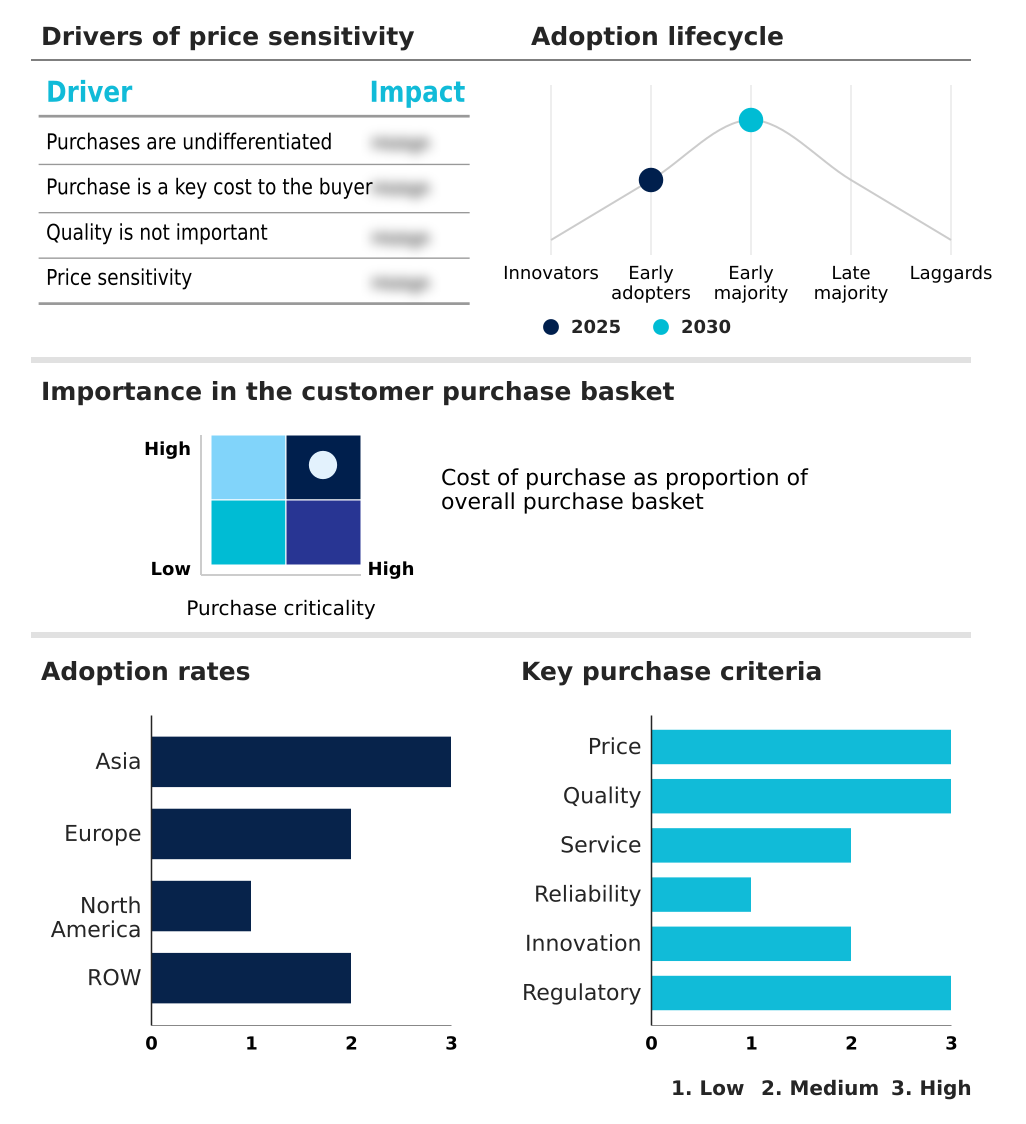
The coronavirus test kits market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the coronavirus test kits market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Coronavirus Test Kits Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, coronavirus test kits market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - The company's offerings include a diverse array of diagnostic solutions, encompassing rapid antigen tests and antibody assays for efficient viral screening and immune response evaluation.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- AdvaCare Pharma
- BGI Group
- Bio Rad Laboratories Inc.
- Biomedomics Inc
- BioMerieux SA
- Cellex Inc.
- Danaher Corp.
- Dynamiker Biotech Co. Ltd.
- F. Hoffmann La Roche Ltd.
- Mylab Discovery Ltd.
- QIAGEN N.V.
- QuidelOrtho Corp.
- Robert Bosch GmbH
- Safecare Biotech Co. Ltd.
- Siemens AG
- Thermo Fisher Scientific Inc.
- Unisys Corp.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Coronavirus test kits market
- In September, 2024, Roche Diagnostics received expanded FDA approval for its cobas 6800/8800 systems to include asymptomatic screening with its multiplex SARS-CoV-2, Flu, & RSV test, broadening its clinical utility.
- In November, 2024, the US Department of Health and Human Services awarded a $150 million contract to Abbott Laboratories to supply Panbio COVID-19 Antigen Self-Tests for the Strategic National Stockpile, reinforcing national pandemic preparedness.
- In January, 2025, Danaher Corp. announced the acquisition of a point-of-care diagnostics startup for $500 million, integrating its rapid molecular testing platform into Cepheid's product line to enhance decentralized testing capabilities.
- In April, 2025, QIAGEN N.V. launched a novel digital PCR (dPCR) assay with single-molecule sensitivity for SARS-CoV-2, designed for high-precision wastewater surveillance and long COVID research applications.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Coronavirus Test Kits Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 289 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Decelerate at a CAGR of -61.7% |
| Market growth 2026-2030 | USD -989.4 million |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | -65.6% |
| Key countries | China, India, Japan, South Korea, Indonesia, Thailand, Singapore, Germany, UK, Russia, France, Italy, The Netherlands, Spain, US, Canada, Mexico, Australia, Brazil, South Africa, UAE, Saudi Arabia and Turkey |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The coronavirus test kits market has evolved into a mature ecosystem focused on managing an endemic pathogen. The strategic imperative for manufacturers has shifted from volume production to value-based innovation, centered on developing sophisticated diagnostic tools.
- Core technologies such as polymerase chain reaction assay, rapid antigen detection, and serological antibody testing are now being integrated into multiplex respiratory panels and advanced point-of-care molecular diagnostics. A key trend is the adoption of a syndromic testing platform, which is a critical boardroom-level consideration influencing R&D and portfolio strategy.
- This shift toward integrated solutions, which can detect sars-cov-2 rna and identify viral proteins, is driven by the need for a more efficient differential diagnosis tool. The implementation of rapid, accurate tests has demonstrated a capacity to reduce hospital processing times by up to 15%.
- This landscape is further shaped by the need for host immune response assays, robust public health surveillance, genomic sequencing for variants, and the maintenance of a strategic national stockpile. As the industry moves forward, success will depend on navigating this complex environment through technological advancement and strategic alignment with new public health priorities.
What are the Key Data Covered in this Coronavirus Test Kits Market Research and Growth Report?
-
What is the expected growth of the Coronavirus Test Kits Market between 2026 and 2030?
-
USD 989.4 million, at a CAGR of -61.7%
-
-
What segmentation does the market report cover?
-
The report is segmented by End-user (Government, and Non government), Type (Rapid test kit, RT-PCR, and Others), Application (Hospitals and clinics, Laboratory and diagnostic centers, and Home care) and Geography (Asia, Europe, North America, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
Asia, Europe, North America and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Endemic status of SARS-CoV-2 and continuous viral evolution, Widespread testing fatigue and declining public demand
-
-
Who are the major players in the Coronavirus Test Kits Market?
-
Abbott Laboratories, AdvaCare Pharma, BGI Group, Bio Rad Laboratories Inc., Biomedomics Inc, BioMerieux SA, Cellex Inc., Danaher Corp., Dynamiker Biotech Co. Ltd., F. Hoffmann La Roche Ltd., Mylab Discovery Ltd., QIAGEN N.V., QuidelOrtho Corp., Robert Bosch GmbH, Safecare Biotech Co. Ltd., Siemens AG, Thermo Fisher Scientific Inc. and Unisys Corp.
-
Market Research Insights
- The market is defined by a strategic realignment toward integrated diagnostics, where syndromic panel adoption is becoming standard practice in clinical settings, improving diagnostic precision for respiratory pathogen co-detection by over 25%. This shift is enabled by decentralized diagnostic solutions, which have broadened healthcare access and reduced patient wait times by an average of 40%.
- However, this evolution is tempered by significant reimbursement policy uncertainty, creating revenue forecasting challenges for manufacturers. The complex regulatory approval transition from emergency authorizations to standard pathways introduces further market friction. Success hinges on navigating these dynamics while delivering products that offer clear clinical decision support, guide effective antiviral treatment guidance, and meet the growing demand for consumer-driven testing.
We can help! Our analysts can customize this coronavirus test kits market research report to meet your requirements.